Additional information
| Water Retention | None |
|---|---|
| Hepatotoxicity | Low risk |
| Lab Test | Not typically required for monitoring |
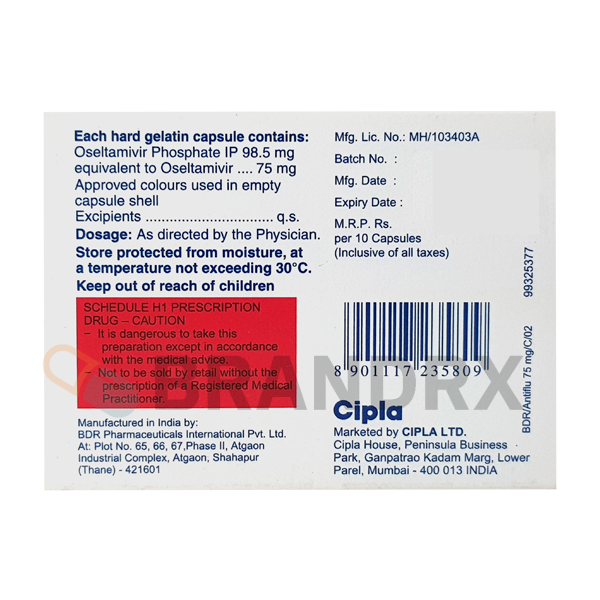
| Strength | 75mg |
| Also known as | Tamiflu |
| Blood pressure | No significant effects |
| Trade name | Tamiflu |
| Storage conditions | Store at room temperature away from moisture and heat |
| Chemical name | (3R,4R,5S)-4-acetylamino-5-amino-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxylic acid, ethyl ester, phosphate (1:1) |
| Formula | C16H28N2O4 В· H3PO4 |
| Substance class | Antiviral |
| Main action | Neuraminidase inhibitor |
| Half-life | 6-10 hours |
| Dosage (medical) | 75 mg twice daily for 5 days |
| Dosage (sports) | Not applicable |
| Effects | Reduces severity and duration of influenza symptoms |
| Side effects | Nausea, vomiting, headache, and rare cases of severe skin reactions and neuropsychiatric events |
| Use in sports | Not typically used in sports |
| Manufacturer | Cipla Ltd. |
| Packing | 10 caps/blister |


Reviews
There are no reviews yet.